Systems medicine analysis of sarcoidosis by targeting mTOR in a co-clinical trial in patients and mice


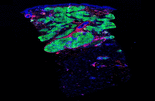
Sarcoidosis is a chronic inflammatory disease of unknown etiology belonging to the group of multisystem granulomatous disorders without efficient targeted therapies. mTOR activation in macrophages was recently identified as a reversible cause of progressive sarcoidosis in a mouse model, raising the exciting prospect of using clinically available mTOR inhibitors in the treatment of systemic sarcoidosis.
In the setting of a clinical trial at the Department of Dermatology, patients with progressive sarcoidosis and skin involvement are sequentially exposed to treatment regimens with the mTOR inhibitor Sirolimus.
The project employs an integrative systems medicine approach to dissect the pathomechanisms of sarcoidosis while evaluating the efficiency of mTOR inhibition.
Time series samples are profiled using single-cell sequencing technologies combined with spatial analysis of immune cell arrangement in lesional skin. Cross-patient bioinformatic analysis is elucidating the pathophysiology of sarcoidosis and mechanisms of action of mTOR inhibition in the disease. Key results are functionally validated in a sarcoidosis mouse model and an inflammatory sarcoidosis skin model with patient-derived inducible pluripotent stem cells.
This project will reveal unknown insights in the pathomechanisms of granulomatous disease while evaluating the efficiency of a new promising treatment modality and validating targets for future clinical studies.
Current project: Systems medicine analysis of sarcoidosis by targeting mTOR in a co-clinical trial in patients and mice
Nikhila <nikhila.harikumar@meduniwien.ac.at>
Current project: Tissue-resident T cells in malignant melanoma
The cutaneous immune system in allogeneic hematopoietic stem cell transplantation

Hematopoietic stem cell transplantation (HSCT) remains a crucial treatment option for malignant diseases of the blood and bone marrow. Its success in curing lymphoproliferative disorders relies on the synergistic effect of eradicating malignant host lymphocytes by pre-transplant treatment regimens and post-transplant graft-versus-leukemia effect (GvL) while limiting severe side effects, namely graft-versus-host disease (GvHD).
In cooperation with the Bone Marrow Transplant Unit (BMT) of the Department of Internal Medicine I of the Medical University of Vienna we analyze the systemic immune response and the cutaneous immune microenvironment in adult HSCT recipients during their treatment and recovery. Furthermore, the unique clinical setting of HSCT is an ideal human model to study basic questions of immune biology: Differentiating host and donor leukocytes on a genomic level allows us to investigate requirements for leukocyte development, survival, function, and migration in vivo.
We found that diverse T-cell responses characterize the different manifestations of cutaneous graft-versus-host disease (Brüggen et al., Blood 2014) and that epidermal elafin expression is an indicator of poor prognosis in cutaneous graft-versus-host disease (Brüggen et al, J Invest Dermatol 2015). Furthermore, we identified the anti-apoptotic protein BCL2 as a potential therapeutic target in steroid refractory GvHD (Strobl et. al, J Invest Dermatol, 2020).
Recently, we showed that host skin-resident memory T cells (TRM) survive radio-chemotherapy and contribute to GvHD development, challenging the paradigm of exclusive graft-versus-host reaction (Strobl et. al, Sci Trans Med 2020).
Our next goal is to deepen our understanding of the immunological processes that occur in the skin during HSCT and GvHD. We are employing state-of-the-art techniques including but not limited to single-cell RNA sequencing, Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq), T cell epitope screening, multicolor flow cytometry and immunofluorescence microscopy to delineate communication axes between immune cells, microbiome and structural cells in the tissue, which ultimately determine the maintenance or breakdown of homeostasis within the skin.
The connection of microbiome dynamics with immune cell regulation in hematopoietic stem cell-transplanted patients
The skin as largest organ is constantly exposed to the environment and home to various microorganisms that influence immune reactions. Patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) suffer from a highly dysregulated microbiome composition due to antibiotics and myeloablative conditioning.
In this project we investigate the long-term impact of the skin and intestinal microbiome on the clinical course after stem cell transplantation including the development of graft-versus-host disease (GVHD). Our approaches include 16S amplicon and whole-meta-genomic shotgun sequencing to study temporal and functional shifts in the bacteria as well as cell culture experiments and 16S rRNA FISH stainings of skin sections. Aside from better understanding the role of the microbiome in inflammation, we hope to identify risk-associated bacterial species linked to survival after HSCT as well as explore additional treatment options such as fecal microbiota transplantation.
Mucosal immunology of HIV infection and its implication on cancer development

People living with HIV (PLWH) have an increased risk of developing non-HIV-related skin cancer, such as melanoma, basal and and squamous cell carcinoma and HPV-related invasive carcinoma. The incidence of these tumors in PLWH is rising along with the increased overall survival thanks to antiretroviral therapy (ART). However, the reason for this increased tumor susceptibility is unclear. We are investigating the skin and mucosal immune environment in PLWH and aim at finding new mechanism for cancer protection in the skin.
Education of mucosal dendritic cells by epithelial cells
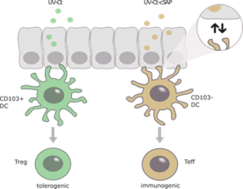
Mucosal protection is critical for prevention and treatment of a broad range of pathogens or neoplasms. We recently showed that mucosal exposure of UV-inactivated Chlamydia trachomatis (UV-Ct) in mice resulted in exacerbated bacterial burden upon subsequent infection. By contrast, mucosal immunization with UV-Ct complexed with charge-switching synthetic adjuvant particles (cSAP) elicited protection. This differential effect was due to the diverse antigen presentation by various dendritic cell subsets.
We currently evaluate the interaction of epithelial cells and dendritic cells in the mucosa of mice and humans upon chlamydia infection and HPV-associated neoplasia. By understanding the mechanisms of the induction of mucosal immunity and tolerance, this project gives insights into new targets for vaccine design against mucosal pathogens and will foster the development of anti-cancer vaccines.
Antigen-specific memory NK cells in health and diseases
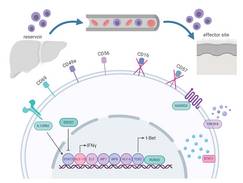
The adaptive immunity is considered an exclusive feature of T and B cells. Historically, NK cells are defined as cells of the innate immune response because they lack RAG-dependent genes. However, evidence suggests that there is a subset of NK cells that can develop long lived and highly specific memory to a variety of antigens in mice and in non-human primates. The existence of human memory cells is still unknown. The aim of this study is to investigate if NK cells of humans can exhibit antigen specific memory responses.
We evaluate phenotype and function of NK cells of liver, blood and skin in detail using state-of-the-art techniques, including FACS analysis, immunofluorescence microscopy functional in vitro killing and migration assays with the ultimate goal to harness memory NK cells for future vaccine designs. We found a subset of NK cells with adaptive functions in the liver and, upon an adaptive immune response, also in the skin (Stary V et al, Sci Immunol 2020). We will now analyze the impact of this NK cell subset on a variety of diseases, including auto-inflammation and infection.
Multi-omics analysis of tissue-specific human Treg signatures in chronic inflammatory diseases
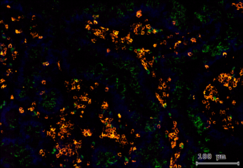
Regulatory T cells (Tregs) are a critical immune component guarding against excessive inflammatory responses with tissue-specific subsets being poised in various organs for direct interference. However, current understanding of Treg heterogeneity and function in non-lymphoid tissue in humans is limited. The goal of this project is to characterize unique types of Treg signatures under steady state and in the context of tissue-confined inflammatory responses in humans using single-cell technologies. The project utilizes samples from patients with psoriasis, sarcoidosis and Crohn's disease, all of which are characterized by a dysregulated inflammatory response in specific organs. We aim to use a multi-omics approach with state-of-the-art techniques to ensure a deeper understanding of Treg subsets in this context at the transcriptional, phenotypical, as well as the metabolic level.
Ultimately, this project will characterize commonalities and differences in signatures of Tregs in the skin and the gut and identify potential molecular targets to modulate immune responses for potential therapeutic purposes.
Functional profiling of stromal cells and their impact on the pathogenesis of granulomatous diseases

A plethora of human diseases of infectious or non-infectious origin feature formation of granulomatous structures in multiple organs and body parts. Granulomas are compact aggregates of immune cell infiltrates, primarily composed of mature macrophages together with other immune cells such as T cells, dendritic cells or plasma cells. Typically, granulomas are surrounded by tissue-resident fibroblastic mesenchymal cells. In fact, in many cases granulomas are circumscribed by concentric scar tissue, which is deposited by fibroblasts.
Tissue scarring is a pathological feature of many granulomatous diseases and poses the main risk for organ transplantation due to lack of targeted therapy and loss of organ function, thus posing a major cause of worldwide morbidity and mortality.
While there is extensive knowledge on the implication of leukocytes in the pathogenesis of granulomatous diseases, the role of non-hematopoietic cells in this process is poorly understood. In this project, we are investigating the role of granuloma-associated fibroblasts (GAFs) on the pathogenesis of granulomatous diseases using unbiased system-wide analyses with single-cell and spatial resolution combined with human tissue bioengineering and high resolution live-cell microscopy. Thereby, this project bridges mechanistic insights with clinical relevance and will contribute to the rational design of effective therapeutic approaches, potentially reaching to other (auto-)inflammatory diseases.
Human immunocompetent skin organoid to mimic inflammatory skin diseases
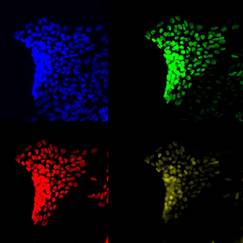
Inflammatory skin diseases represent a considerable amount of the global burden of disease and the identification of their pathogenesis is still limited due to the absence of a proper human skin model that mimics native tissue of healthy and especially diseased individuals. The vast heterogeneity within cell types and the complex and dynamic inflammation site in different skin diseases have enormous impact on treatment responses. Current in vitro skin equivalents are not complex and mature enough to serve as disease models.
In this project we aim to establish a complex human skin organoid in the presence of immune cells and blood vessels as a relevant model to study cellular interactions and metabolic aspects in skin diseases. They are comprised of primary cells from skin biopsies and patient derived induced pluripotent stem cells (iPSCs). A combination of direct differentiation, bio printing, 3D cell culture and the manipulation of molecules and signaling pathways enables us to mimic and study cellular processes involved in inflammatory skin diseases and may give rise to novel strategies for personalized medicine.
(LBI-RUD) Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases (LBI-RUD)

The LBI-RUD has been founded to implement a coordinated research program on the genetic and functional basis of rare diseases. Rare diseases are paradigmatic for the upcoming era of personalized and participatory medicine, and LBI-RUD broadly embraces its pioneering role for the future of medicine with the vision to provide an internationally visible contribution to its realization. The LBI-RUD partners Medical University of Vienna (MedUni Vienna), with the largest university hospital in Europe, the St. Anna Children’s Cancer Research Institute (CCRI), and the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, provide an ideal interface to bridge state-of-the-art research and clinical practice. With their support, LBI-RUD has been deeply integrated into the national network to diagnose, characterize, and treat rare genetic defects as an active part within the Vienna Center for Rare and Undiagnosed Diseases (CeRUD).
The Stary group at LBI-RUD focuses on the characterization of host-pathogen interactions in the skin and on mucosal membranes. The combination of high-level clinical research with human tissue as well as mouse models are ideally suited to successfully translate research results to clinics.
More information can be found on the website of the LBI-RUD.
