Adaptive immunity in cutaneous Lyme disease
Skin infection with tick-borne Borrelia burgdorferi causes multistage clinical manifestations that include dermatological and neurological involvement and may result in a chronically infected state in the untreated individual. However, the specific mechanisms contributing to lack of memory formation, multisystem disease and persistent infections despite significant immune responses remain elusive. To address this issue, we use a systematic multi-omics immune cell profiling approach: Single-cell sequencing technologies of skin samples from affected patients and an ex vivo tick bite model with artificial introduction of spirochetes allow us to perform systems biology studies on the primary immune response of innate and adaptive immune cells and factors leading to lack of bacterial clearance. Overall, this translational and patient-oriented study aims at deciphering immunosuppressive effects of tick saliva and immunological mechanisms that are exploitable for treatment and/or prevention of Lyme disease and other tick-borne infections.
Tissue-resident memory T cell diversity in oral mucosa
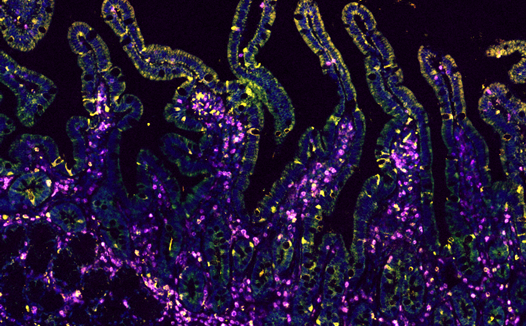
A reliable, long-lasting protection against viral infections including SARS-CoV-2 is based on the generation of memory T cell populations within the affected tissues. As one of the first sites of contact with infectious aerosols, the assessment of specific T cell subpopulations in the oral mucosa is of utmost interest for the evaluation of adaptive cellular immune responses to vaccinations or natural infections. We investigate T cell receptor diversity in of oral mucosal of healthy individuals after viral infections, to dissect diversity of virus-specific tissue-resident memory T cells. This ongoing project may contribute to further understanding of T cell responses, tissue-resident T cell longevity, and retention of T cells at effector sites following viral contact.
Resident immunity in graft-versus host disease
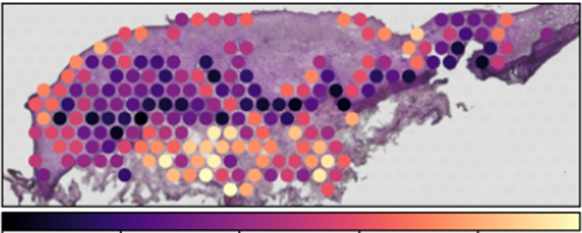
Graft-versus-host disease (GvHD) remains a major complication following allogeneic hematopoietic stem cell transplantation, frequently affecting barrier tissues such as the skin and gastrointestinal tract. While systemic immune activation is well-characterized, the role of host- and donor-derived tissue-resident immune cells in driving local inflammation and tissue damage remains unclear. In this project, we dissect the functional phenotypes, clonal architecture, and local interactions of immune cells in affected tissues. Our goal is to define key immunopathological circuits propagated by tissue-resident immune cells of host and donor origin that contribute to chronic inflammation and tissue injury in acute and chronic GvHD. Ultimately, this translational research aims to identify novel therapeutic targets to improve the management of GvHD in transplant recipients.
