Investigating the role of the extracellular matrix to induce “pioneer melanoma cells”
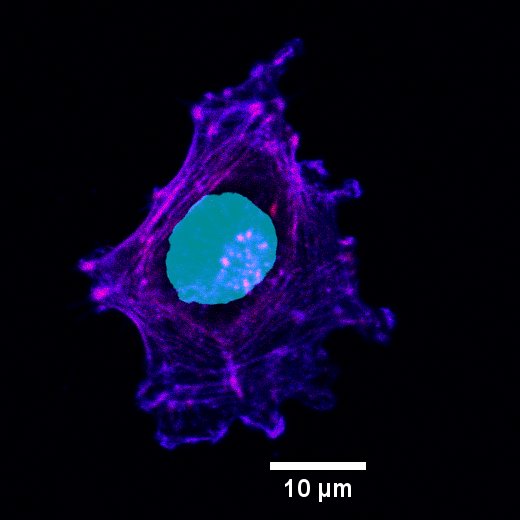
Cancer metastasis is the reason why later stages of melanoma are closely associated with poor patient prognosis. To metastasize, cells migrate from the initial tumor into the surrounding tissue and even distal sites in the body. Cells sense and interact with the extracellular matrix, mainly through cell protrusions called filopodia. To migrate, epithelial melanoma cells undergo various changes to transdifferentiate into a mesenchymal phenotype, enabling migration and metastasis. From a primary tumor, few initial highly migratory cells, pioneer melanoma cells, are hypothesized to initiate invasion of the surrounding tissue. The migration tracks that form in the fibers of the extracellular matrix enable cells to follow the initial pioneer cancer cells, thereby facilitating metastasis formation.
The project aims to identify the changes cells undergo when switching from an epithelial to mesenchymal phenotype. In this project, we focus on detecting the interaction of cancer cells with their environment which plays a crucial role in invasiveness.
Collagen matrices of varying stiffness are used to mimic the native environment of melanoma cells in the skin. Through live cell imaging of these cells in 3D matrices, we may better understand the mechanisms behind their invasive behavior. Further, gene expression will be analyzed to investigate the epithelial-to-mesenchymal transition at the molecular level.
People involved in the projects: Karin Pfisterer (Principal Investigator), Pauline Weinzettl
