Broad-spectrum RG1-VLP vaccine phase I human trial
To develop a broadly protective HPV vaccine we have combined L1 VLP with the conserved protective epitope of L2 defined by the RG-1 monoclonal antibody. This experimental RG1-VLP vaccine has been produced under cGMP supported by the NIH PREVENT Cancer program. We have gained patents and NCI CP-CTNet support for a Phase I study in the US and Austria, and an FDA ‘safe to proceed’ notice.
Prevention of non-melanoma skin cancer in immunosuppression
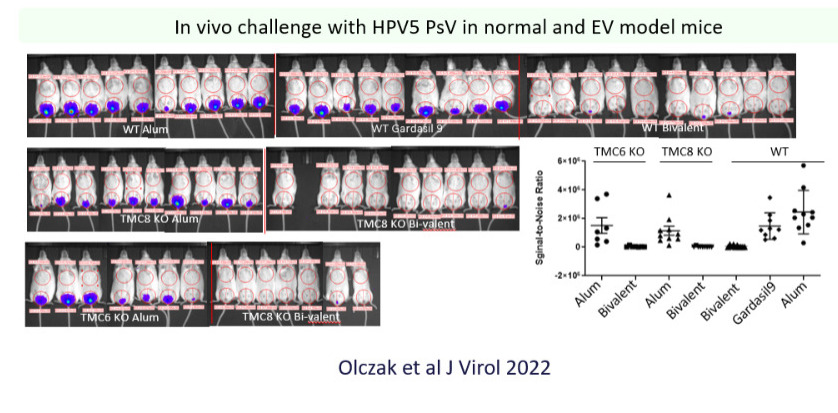
Cutaneous HPV types commonly causing skin warts in children and particularly in immunosuppressed patients are not targeted by current vaccines, as are species beta HPV that play an adjunct role in the development of non-melanoma skin cancer (squamous cell and basal cell cancers) in immunosuppressed patients. We aim to develop prophylactic vaccines against cutaneous HPV types based on chimeric L1-L2 VLP. If proven effective in animal models, this may provide a rational to immunize children with a next-generation vaccine that protects against skin warts, anogenital and oropharyngeal cancers, and may even reduce development of non-melanoma skin cancers.
Measles-virus based papillomavirus vaccines
Measles vaccine is widely used, inexpensive, induces robust protective immunity, has an excellent safety record especially as a pediatric vaccine, and has been tested as vaccine platform against other viruses such as Chikungunya, Hepatitis B/C, Dengue, or SARS-2 Coronavirus. We plan to capitalize on these properties and generate an economic vaccine candidate that protects against all cancer-causing HPV types. Measles virus platform derived vaccines have high potential to lower cost burden to public health care, an important aspect particularly to reach low- and middle-income countries. Such vaccines could be implemented at no extra costs in the routine WHO measles eradication program, since the vaccine candidate is still protective against measles. Vaccinations would also protect both sexes against genital HPV infection when administered before reaching adolescence (“buy one, get one free”). This strategy offers tremendous potential for universal HPV coverage by inclusion into children routine vaccination programs and also target skin warts and other HPV-related complications.
Thermostability of a broad-spectrum HPV vaccine
Delivering an effective HPV vaccine to developing countries comes with many challenges. For example, keeping vaccines at a temperature sufficient to maintain the composition and reduce degradation can be difficult when delivering vaccines to remote regions and limited refrigerated space is available for vaccine storage. We aim to lyophilize or freeze-drye the broad-spectrum HPV vaccine formulation in the presence of a hypertonic mixture to increase stability or decrease degradation or disassembly of the constructs during storage, transportation, delivery resulting in a reduction of product loss and reduction of loss of efficacy.
Atomic layer deposition (ALD) prime-boost vaccine formulation
Delivery of HPV vaccines in economically disadvantaged countries, which carry 80% of the worldwide burden of cervical cancer, is complicated by the vaccines’ costs and the need for three immunizations. We aim to generate a next-generation thermostable, single-shot, prime-boost microparticle vaccine to protect against a broad-spectrum of HPV types. In collaboration with University of Collorado at Boulder, antigen/adjuvant formulations containing glass-forming polymers and trehalose are first are spray-dried to form glassy microparticles that confer thermostability. Atomic layer deposition (ALD) reactions conducted in fluidized beds are then used to coat the microparticles with defined numbers of molecular layers of alumina that modulate the timed release of the internalized antigen and act as adjuvant. Single doses of the ALD-coated vaccine formulations will elicit a prime-boost immune response, that produce neutralizing responses equivalent or superior to conventional prime-boost doses of liquid formulations.