Recent and Ongoing Projects
- Formation and function of the prenatal human skin immune system
- Involvement of the endoplasmic reticulum-associated protein reticulon 1A in cutaneous immunity
- Establishment and application of ex vivo skin models
- Effects of hydrogels containing antiseptics and other compounds for wound care
1. Formation and function of the prenatal human skin immune system – role of T cells in developing skin
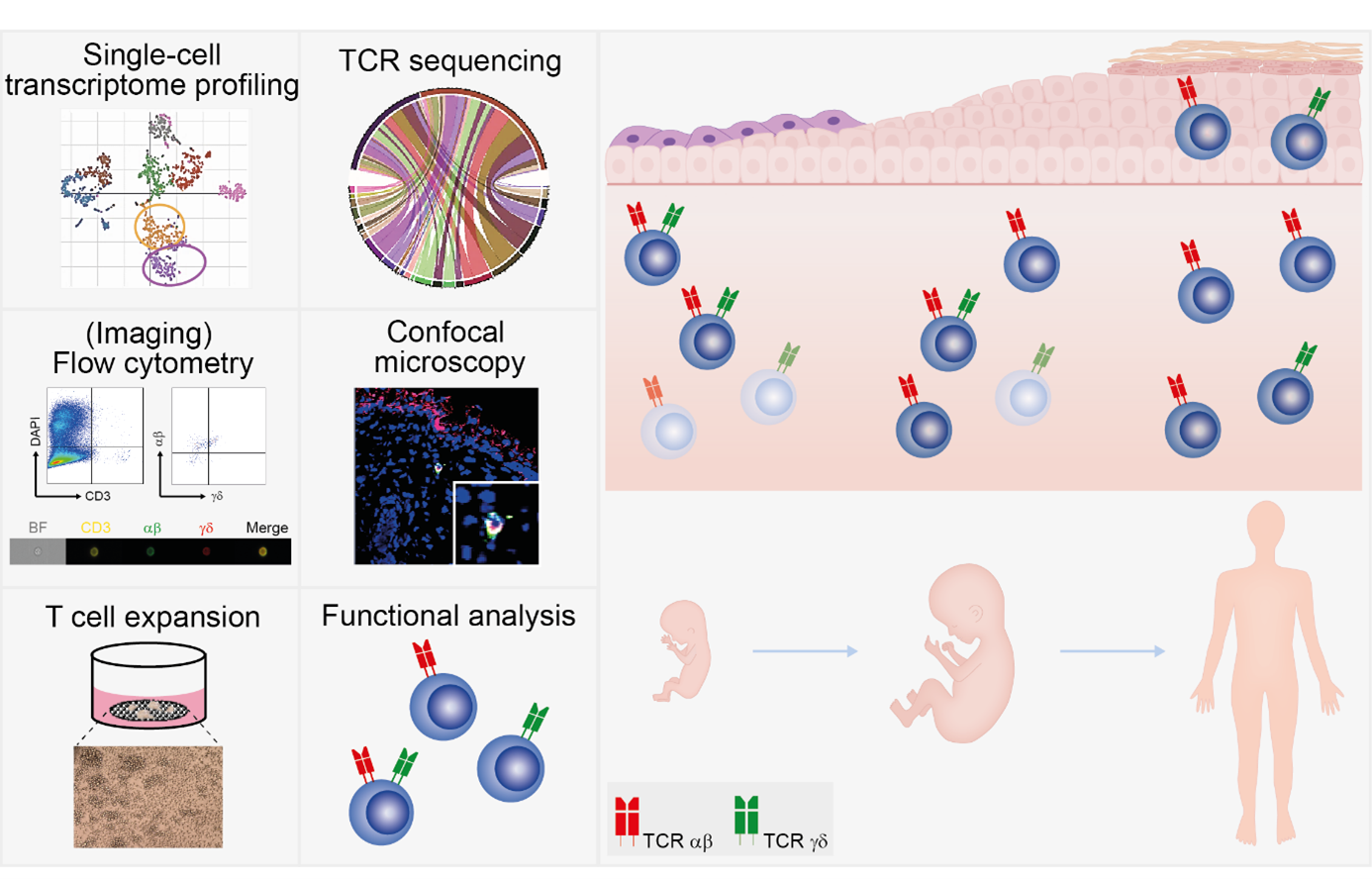
As the outermost organ of the body, mammalian skin prevents pathogen entry as well as protects against damage from the environment. The three skin compartments (epidermis, dermis and hypodermis) function as physical/mechanical, chemical and immune barriers. The latter employs a complex network of immune cell types of the myeloid lineage (macrophages, dendritic cells, and mast cells) as well as lymphoid lineage (T cells, and innate lymphoid cells). Immune cells form an interactive network with non-immune cells, which regulate tissue residency, recruitment, and activation of immune cells to maintain skin homeostasis. Work over the last two decades has highlighted the importance of T cells in adult human skin. They detect antigens through heterodimeric T cell receptors (TCRs) composed of either α and β or γ and δ chains and are involved in the defense against pathogens and tumors, play a role in tissue homeostasis but can also cause inflammation and autoimmune diseases. T cells are present in fetal skin. However, our understanding of their contribution to early immunity and morphogenesis were incomplete owing to the lack of comprehensive methodologies for the assessment of extremely rare starting T cell numbers, lack of dissociation methods that liberate viable T cells with high yield and preserved T cell markers and by the limited amount of and access to fetal human tissue. To bypass these limitations, we used a combination of modern and conventional laboratory techniques (transcriptome analysis by droplet-based single-cell RNA sequencing technology, TCR repertoire profiling, multidimensional (imaging) flow cytometry, multiplex cytokine assays, in situ immunofluorescence) and explored the complexity and function of fetal skin T cells. Moreover, we developed an in vitro platform to propagate these cells which recapitulated the identities of their in vivo counterparts. We identified a distinctive T cell population co-expressing αβ and γδ TCRs in fetal skin and intestine but not in other fetal organs and peripheral blood. Double-positive (DP) αβγδ T cells and single-positive (SP) αβ T cells with a naive phenotype are the predominant populations in fetal skin followed by discrete subsets of SP γδ T cells, memory and regulatory T cells. Of note, the DP αβγδ T cell population is unique to the prenatal period and is absent in the skin at the time of birth and in healthy adults. Importantly, DP αβγδ T cells display little overlap of complementary-determining region 3 (CDR3) sequences with SP αβ T cells. Gene signatures, cytokine profiles and in silico receptor-ligand interaction studies indicate their contribution to early skin morphogenesis. DP αβγδ T cells are antigen-responsive in vitro, suggesting their participation in the protection of the fetus against pathogens in intrauterine infections. Collectively, this unique cutaneous T cell type within the native fetal skin microenvironment points to fundamental differences in the immune surveillance between fetal and adult human skin and advance the understanding of skin immunity in early life.
Selected Publications
R. Reitermaier, T. Ayub, J. Staller, P. Kienzl, N. Fortelny, P.A. Vieyra-Garcia, C. Worda, C. Fiala, C. Staud, W. Eppel, A. Scharrer, T. Krausgruber, A. Elbe-Bürger: The molecular and phenotypic makeup of fetal human skin T lymphocytes. Development 2022 Apr 15;149(8):dev199781. doi: 10.1242/dev.199781. Epub 2021 Oct 26
R. Reitermaier, T. Krausgruber, N. Fortelny, T. Ayub, P.A. Vieyra-Garcia, P. Kienzl, P. Wolf, A. Scharrer, C. Fiala, M. Kölz, M. Hiess, M. Vierhapper, C. Schuster, A. Spittler, C. Worda, W. Weninger, C. Bock, W. Eppel, A. Elbe-Bürger: αβγδ T cells play a vital role in fetal human skin development and immunity. J Exp Med. 2021 Apr 5;218(4):e20201189. doi: 10.1084/jem.20201189.
C. Schuster, M. Mildner, A. Botta, L. Nemec, R. Rogojanu, L. Beer, C. Fiala, W. Eppel, W. Bauer, P. Petzelbauer, A. Elbe-Bürger: Development of blood and lymphatic endothelial cells in embryonic and fetal human skin. Am. J. Pathol. 185:2563-2574, 2015.
C. Schuster, M. Mildner, M. Mairhofer, W. Bauer, C. Fiala, M. Prior, W. Eppel, A. Kolbus, E. Tschachler, G. Stingl, A. Elbe-Bürger: Human embryonic epidermis contains a diverse Langerhans cell precursor pool. Development 141:807-815, 2014.
M. Mildner*, M. Prior*, M. Gschwandtner, C. Schuster, E. Tschachler, A. Elbe-Bürger: Epidermal CCL27 expression is regulated during skin development and keratinocyte differentiation. J. Invest. Dermatol. 134:855-858, 2014. *share first authorship
N. Iram, M. Mildner, M. Prior, P. Petzelbauer, C. Fiala, S. Hacker, E. Tschachler, A. Elbe-Bürger: Age-related changes in expression and function of Toll-like receptors in human skin. Development 139:4210-4219, 2012.
C. Schuster, C. Vaculik, M. Prior, C. Fiala, M. Mildner, W. Eppel, G. Stingl, A. Elbe-Bürger: Phenotypic characterization of leukocytes in prenatal human dermis. J. Invest. Dermatol. 132:2581-2592, 2012.
C. Schuster, C. Vaculik, C. Fiala, S. Meindl, O. Brandt, M. Imhof, G. Stingl, W. Eppel, A. Elbe-Bürger: HLA-DR+ leukocytes acquire CD1 antigens in embryonic and fetal human skin and contain functional antigen-presenting cells. J. Exp. Med. 206:169-181, 2009
2. Involvement of the endoplasmic reticulum-associated protein reticulon 1A in cutaneous immunity
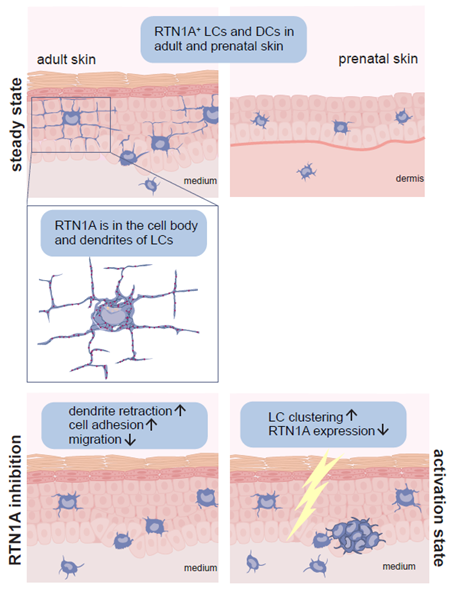
The skin is serving as an important barrier against the harsh extrinsic environment and is equipped with diverse defense mechanisms. Among others, multiple phenotypically and spatially distinct dendritic cell (DC) populations such as epidermal Langerhans cells (LCs) and dermal DCs fight external intruders and orchestrate immune responses. Their nature and function is not fully understood. Recently, we identified that LCs and dermal DCs as well as their precursors in adult and fetal human skin express the endoplasmic reticulum-associated protein reticulon 1A (RTN1A). RTN1A expression was detected also in human LC-surrogate in vitro models such as MUTZ-3-LCs and LCs differentiated from CD34+ cord-blood progenitor cells. Ontogeny studies in mice revealed expression of RTN1A in LC precursors and in developing nerves in prenatal skin. In adult mice, distinct populations of conventional DCs in primary and secondary lymphoid organs and epidermal LCs but not dermal DCs expressed RTN1A. Functional experiments demonstrated that RTN1A inhibited calcium flux and affected cell morphology and size in THP-1 cells overexpressing RTN1A. Further, we discovered a relation between activation of Toll-like receptors, clustering of LCs, and downregulation of RTN1A in human epidermal explants ex vivo, thus indicating an important role of RTN1A in LC residency and maintaining tissue homeostasis.
Publications
M.A. Cichoń, K. Pfisterer, J. Leitner, L. Wagner, C. Staud, P. Steinberger, A. Elbe-Bürger: Interoperability of RTN1A in dendrite dynamics and immune functions in human Langerhans cells. Elife. 2022 Oct 12;11:e80578. doi: 10.7554/eLife.80578.
M.A. Cichoń, K. Klas, M. Buchberger, M. Hammer, K. Seré, M. Zenke, E. Tschachler, A. Elbe-Bürger: Distinct distribution of RTN1A in immune cells in mouse skin and lymphoid organs. Front Cell Dev Biol. 2021 Jan 15;8:608876. doi: 10.3389/fcell.2020.608876. eCollection 2020.
M. Gschwandtner*, P. Kienzl*, P. Tajpara, C. Schuster, G. Stipek, M. Buchberger, M. Mildner, M. Mairhofer, W. Eppel, M. Vierhapper, J. Pammer, R. Koller, A. Elbe-Bürger§, E. Tschachler§: The reticulum-associated protein RTN1A specifically identifies human dendritic cells. J. Invest. Dermatol. 2018 Jun;138(6):1318-1327. doi: 10.1016/j.jid.2018.01.002. Epub 2018 Jan 31. share*first or §corresponding authorship
3. Establishment and application of human ex vivo skin models
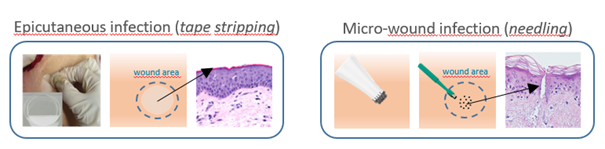
A large body of literature is available on wound healing in humans. As a standardized ex vivo wound model without disruption of the dermal compartment has not been put forward with compelling justification we established a novel ex vivo wound model based on application of negative pressure. Importantly, the basement membrane remained intact after blister roof removal and keratinocytes were absent in the wounded area. Upon six days of culture, the wound was covered with one to three-cell thick K14+Ki67+ keratinocyte layers, indicating that proliferation and migration were involved in wound closure. After eight to twelve days, a multi-layered epidermis was formed expressing epidermal differentiation markers. Together, our model recapitulates the main features of epithelial wound regeneration, and can be applied for testing wound healing therapies and investigating underlying mechanisms in the future.
Together with keratinocytes (KCs) and Langerhans cells (LCs), the epidermis is an ideal portal for vaccine delivery. Pattern recognition receptor agonists, in particular polyinosinic-polycytidylic acid [p(I:C)], are promising adjuvant candidates for therapeutic vaccination to generate protective T cell immunity. To study the expression and activation of double-stranded RNA (dsRNA)-sensing pattern recognition receptors in LCs and KCs in human skin we established an ex vivo skin explant model using tape stripping. Whereas KCs expressed all known dsRNA sensing receptors at a constitutive and inducible level, LCs exclusively expressed melanoma differentiation-associated protein 5 (MDA5) in untreated skin and freshly isolated cells. Comparative assessments of downstream signaling pathways induced by p(I:C) revealed distinct mitochondrial antiviral-signaling protein, IFN-regulatory factor 3, and NF-κB activation in LCs and KCs. Stimulation of freshly isolated LCs with specific ligands revealed that only the MDA5-specific ligand induced IFN-α2, IFN-β, and TNF-α cytokines while in KCs, both TLR3 and MDA5-specific ligands induced production of high IL-6 and IL-8 levels, and low IFN-α2 and IFN-β levels, indicating that different dsRNA-sensing receptors and/or downstream signaling pathways are activated. Our data suggest that MDA5 may be an attractive adjuvant target for epicutaneous delivery of therapeutic vaccines with the goal to target LCs.
Herpes simplex virus (HSV) infections can cause considerable morbidity. Currently, nucleoside analogues such as acyclovir are widely used for treatment. However, HSV infections resistant to these drugs are a clinical problem among immunocompromised patients. To provide more efficient therapy and to counteract resistance, a different class of antiviral compounds has been developed. Pritelivir, a helicase primase inhibitor, represents a promising candidate for improved therapy. We established an HSV-1 infection model on microneedle-pretreated human skin ex vivo and identified HSV-1 specific histological changes, down-regulation of nectin-1, nuclear translocation of NF-kB, interferon regulatory factor 3, and signaling of the IFN-inducible protein MxA. Accordingly, this model was used to test the potency of pritelivir compared with the standard drug acyclovir. Both drugs had a comparable efficacy for inhibiting HSV-1 replication, suggesting that pritelivir could be an alternative therapeutic agent for patients infected with acyclovir-resistant strains. The use of bona fide human skin to examine virus-cell interactions after infection is not only a powerful step forward to understanding and countering the pathogenesis of HSV or other viral infections but also provides a platform for future development and testing of antiviral drugs.
Selected Publications
A. Rakita, N. Nikolić, M. Mildner, J. Matiasek, A. Elbe-Bürger: Re-epithelialization and immune cell behaviour in an ex vivo human skin model. Sci. Rep. 2020 Jan 8;10(1):1. doi: 10.1038/s41598-019-56847-4.
P. Tajpara, M. Mildner, R. Schmidt, M. Vierhapper, J. Matiasek, T. Popow-Kraupp, C. Schuster, A. Elbe-Bürger: A preclinical model for studying herpes simplex virus infection. J. Invest. Dermatol. 2018 Nov 8. pii: S0022-202X(18)32794-5. doi: 10.1016/j.jid.2018.08.034. Epub 2018 Nov
P. Tajpara, C. Schuster, E. Schön, P. Kienzl, M. Vierhapper, M. Mildner§, A. Elbe-Bürger§: Epicutaneous administration of the pattern recognition receptor agonist polyinosinic-polycytidylic acid activates the MDA5/MAVS pathway in Langerhans cells. FASEB J. 2018 Aug;32(8):4132-4144, 2018; doi: 10.1096/fj.201701090R. Epub 2018 Mar 6. §share corresponding authorship
4. Effects of hydrogels containing antiseptics and other compounds for wound care
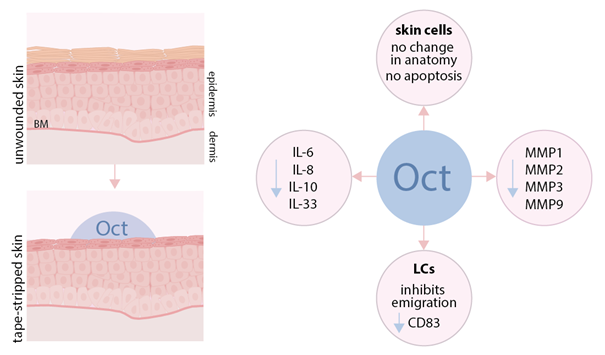
Wound healing is a crucial process for maintaining the function of human skin as a protective barrier to pathogens and other external stress factors. Hydrogels – in combination with antimicrobials – are often used, as moist wound care has been widely accepted as standard therapy. We have comparatively assessed the mechanism of action of several commercially available hydrogels for wound care that contain antiseptic molecules [e.g. octenidine (octenilin®), poyhexanide (Prontosan®, Lavanid®), PVP-Iodine (Betadona®) and products based on sodium hypochlorite / hypochlorous acid (ActiMaris®, Microdacyn60®, VeriforteTMmed)]. Hence, tape-stripped human ex vivo skin biopsies were treated topically with wound gels and cultured for 48 h. Haematoxylin & eosin as well as caspase-3 staining of treated biopsies showed that octenilin® does not alter skin morphology and shows the least interfering effect on human epidermal cells compared to untreated controls. octenilin® prevented the emigration of LCs and inhibited the upregulation of maturation and costimulatory markers. Further, enzyme-linked immunosorbent assays and an enzyme activity assay of culture supernatants revealed that octenilin® demonstrates significantly broader anti-inflammatory and protease-inhibitory capacities than other wound gels. Overall, this study clearly demonstrates totally different effects for several commercially available hydrogels in our wound model, which gives also new insight into their tissue compatibility and mode of action.
Publications
S. Seiser, D. Cerbu, A. Gallhofer, J. Matiasek, A. Elbe-Bürger: Comparative assessment of commercially available wound gels in ex vivo human skin reveals major differences in immune response-modulatory effects. Sci Rep. 2022 Oct 19;12(1):17481. doi: 10.1038/s41598-022-20997-9.
S. Seiser, L. Janker, N. Zila, M. Mildner, A. Rakita, J. Matiasek, A. Bileck, C. Gerner, V. Paulitschke, A. Elbe-Bürger: Octenidine-based hydrogel shows anti-inflammatory and protease-inhibitory capacities in wounded human skin. Sci Rep. 2021 Jan 8;11(1):32. doi: 10.1038/s41598-020-79378-9.
N. Nikolić, P. Kienzl, P. Tajpara, M. Vierhapper, J. Matiasek, A. Elbe-Bürger: The antiseptic octenidine inhibits Langerhans cell activation and modulates cytokine expression upon superficial wounding with tape stripping. J. Immunol. Res. 2019 Mar 3:2019:5143635. doi: 10.1155/2019/5143635. eCollection 2019.
